
Programs & Research
Our pipeline diversification offers opportunities to advance novel treatments across therapeutic areas and mechanisms of action
MM120 | LSD D-tartrate
Lead Candidate with Evidence Across Multiple Therapeutic Areas
Extensive evidence of clinical benefit and mechanistic rationale in psychiatry, pain and substance use disorders
Learn more about the study at our Clinical Trials page.
MM402 | R(-)-MDMA
Clinical Data Support Opportunity in ASD
Pilot clinical trial results of MDMA demonstrate acute and durable positive effects on social functioning in ASD population
1. Pitts 2018; Psychopharmacology; 235.
2. Curry 2018; Neuropharmacology; 128.
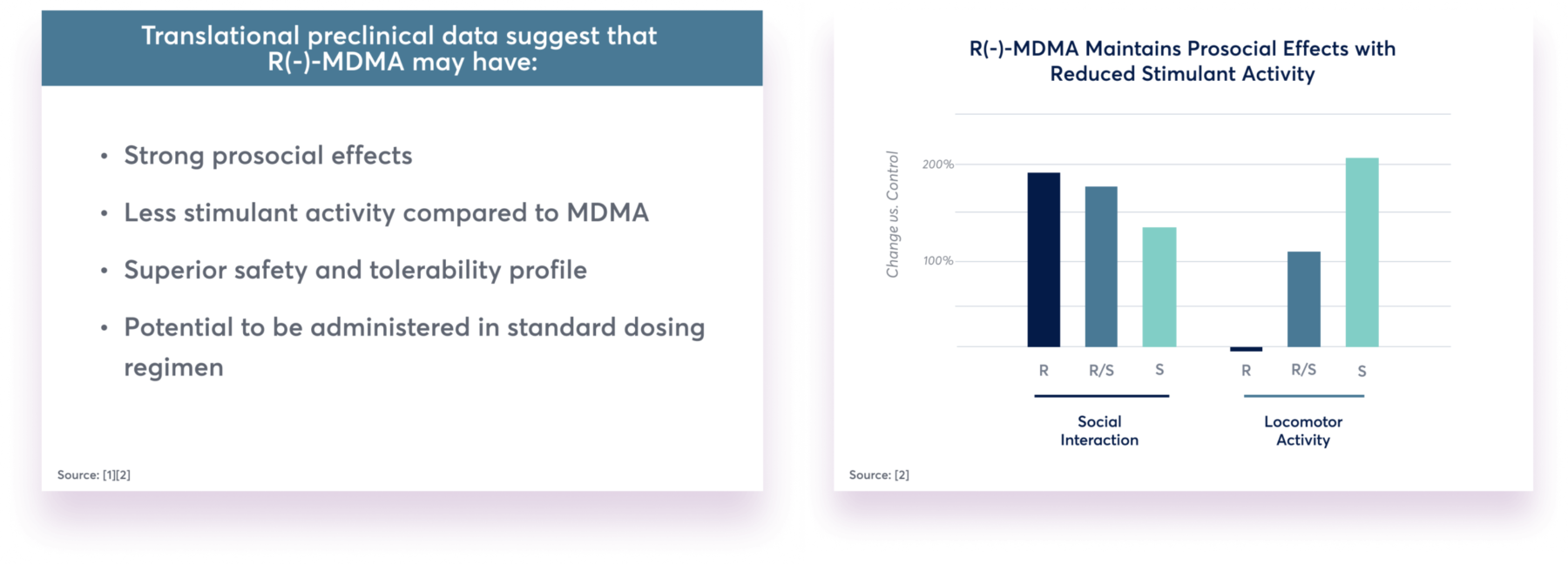
Translational preclinical data suggest that R(-)-MDMA may have:
- Strong prosocial effects
- Less stimulant activity compared to MDMA
- Superior safety and tolerability profile
- Potential to be administered in standard dosing regimen
Relevant References, Publications and Scientific Literature
- NIMH Mental Health Information (NIMH 2020; Mental Illness.)
- Mental and Substance Use Disorders Prevalence Study (MDPS): Findings Report (RTI International 2023)
- Epidemiology of anxiety disorders in the 21st century (Bandelow 2015; Dialogues Clin. Neurosci; 17(3).)
- Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States (Leigh & Du 2015; J. Autism Dev. Disord.; 45(12).)
- Safety and Efficacy of Lysergic Acid Diethylamide-Assisted Psychotherapy for Anxiety Associated With Life-threatening Diseases (Gasser 2014; J. Nerv. Ment. Dis.; 202(7).)
- Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials (Fuentes 2020; Front Psychiatry; 10:943.)
- Dark Classics in Chemical Neuroscience: Lysergic Acid Diethylamide (LSD) (Nutt 2020. Cell; 181(1).)
- The pharmacology of lysergic acid diethylamide: a review (Nichols 2018.)
- Psychedelic Psychiatry’s Brave New World (Nutt 2020. Cell; 181(1).)
- Psychedelics in the treatment of unipolar mood disorders: a systematic review (Rucker 2016. J. Psychopharmacol; 30(12).)
- Lysergic acid diethylamide-assisted therapy in patients with anxiety with and without a life-threatening illness: A randomized, double-blind, placebo-controlled Phase II study (Holze, Gasser et. al 2022. Biological Psychiatry.)
- The Prevalence of Parent-Reported Autism Spectrum Disorder Among US Children (Kogan 2018)
- Autism Patient Focused Drug Development Meeting Slides (FDA Patient Focused Drug Development workshop on Autism Spectrum Disorder (2017))
- MDMA-assisted therapy: A new treatment model for social anxiety in autistic adults (Danforth 2018; Psychopharmacology; 235.)
- (±)-MDMA and its enantiomers: potential therapeutic advantages of R(-)-MDMA (Pitts 2018; Psychopharmacology; 235.)
- Separating the agony from ecstasy: R(e)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice (Curry 2018; Neuropharmacology; 128.)
NOTE: Information above was obtained form publicly available sources and is presented only for purposes of providing a general overview of information on investigational products and uses that have not been approved by the U.S. Food and Drug Administration.